Bioanalytical
Immunogenicity
Kanwhish Biotech has extensive experience in immunogenicity analysis for preclinical studies, clinical trials, and post-marketing surveillance. We offer comprehensive and multi-dimensional immunogenicity evaluation services. We provide reliable assessments of humoral immune responses (ADA, NAb), cellular immune reactions, and innate immune response and cytokine release analyses. Adverse immune reactions are generally caused by humoral immune responses. Therefore, immunogenicity studies primarily focus on the detection and characterization of anti-drug antibodies (ADA) and neutralizing antibodies (NAb). Our team of professional scientists can integrate and analyze immunogenicity data for complex drugs and provide practical recommendations for your product.
| Immunogenicity analysis may involve humoral immune responses (ADA/NAb), cellular immune reactions, and detection of innate immune factors; |
| Analytical method platforms include ligand binding assays (LBA) and cell-based assays (CBA); |
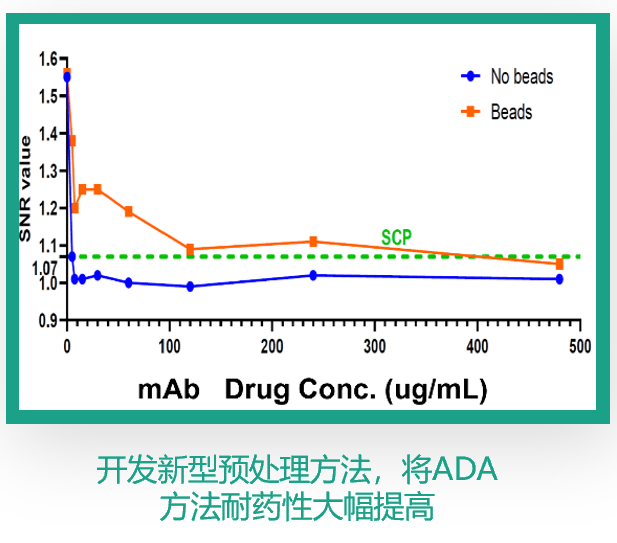
| Professional ADA/NAb method development & validation, sample data analysis; |
| Capable of meeting global regulatory requirements for immunogenicity analysis methods; |
| Method validation and sample testing conducted to meet regulatory filing requirements; |
| Extensive experience in regulatory filing preparation related to bioanalysis and can assist with immunogenicity data analysis. |
Please tell us your questions or needs
and Kanwhish Biotech will contact you as soon as possible via email or phone.

 © 2022-2025 Copyright All Rights Reserved. 苏ICP备2023013734号-1
© 2022-2025 Copyright All Rights Reserved. 苏ICP备2023013734号-1